ADDIN Series
ADDIN- Unique PMS-EDC system developed in-house by CRO -
"ADDIN" is a unique EDC system for PMS developed in-house by CRO.
It provides One-stop Support System shared by both CRO and vendors, with a rapid collection function for adverse drug events and PV related information.
With 20 years of evolutionary history with "ADDIN" and "ADDIN EX", current “ADDIN SMALLT” is highly evaluated by doctors and outsourcers with its easy-to-use operability and workflow suitable for PMS.
With 20 years of evolutionary history with "ADDIN" and "ADDIN EX", current “ADDIN SMALLT” is highly evaluated by doctors and outsourcers with its easy-to-use operability and workflow suitable for PMS.
With 20 years of evolutionary history with "ADDIN" and "ADDIN EX", current “ADDIN SMALLT” is highly evaluated by doctors and outsourcers with its easy-to-use operability and workflow suitable for PMS.
Service Features
- Point 1.
- EDC system for PMS developed in-house by CRO
- Point 2.
- User-friendly EDC screen easy to operate for Medical Doctors
- Point 3.
- Extensive experience and achievements of consigned projects

|image scroll
Main Services with Details
Basic functions/features
Wide Variety of Data Interactions
ADDIN has the following data management functions to manage large amounts of data management for PMS
- Coding (disease, drug name, adverse event)
- Logical Check (Secondary logical)
- Reexamination Request Form preparation
- Data Management Task Control (including progress follow-up function)
Email Transmission Function
The messaging function in ADDIN supports smooth and reliable delivery of survey/resurvey requests via email.
Record Tracking Functions
(Audit Trail Functions)
ADDIN manages data from initial data entry by medical doctors to the final lock. The recorded data is searchable for confirmation and output.
Survey Specification Changes and Version Upgrades
Changes in survey items and logical check specifications often occur during PMS; however, such specifications and content changes are possible without suspension of the survey.
Electronic Signature Function
The messaging function in ADDIN supports smooth and reliable delivery of survey/resurvey requests by emails.
Optional functions/features
Linkage to external interface
- Progress Follow-up System (ADDIN.SCOPE, POSTIER, PostMa Watch, ClipCapture)
- Safety Database system (Argus, ARISj, ClinicalWorks, Perceive) *EtoB connection
- ePRO system
Capture PDF output
- Convert the screen image of the survey form to PDF for output
Batch logical function
In case the reexamination criteria are revised during operation, received data can be checked with the latest reconciliation standards.
One-stop Support System shared by both CRO and vendors
Smooth, stress-free operation throughout the processes from EDC setup to PMDA application
|image scroll
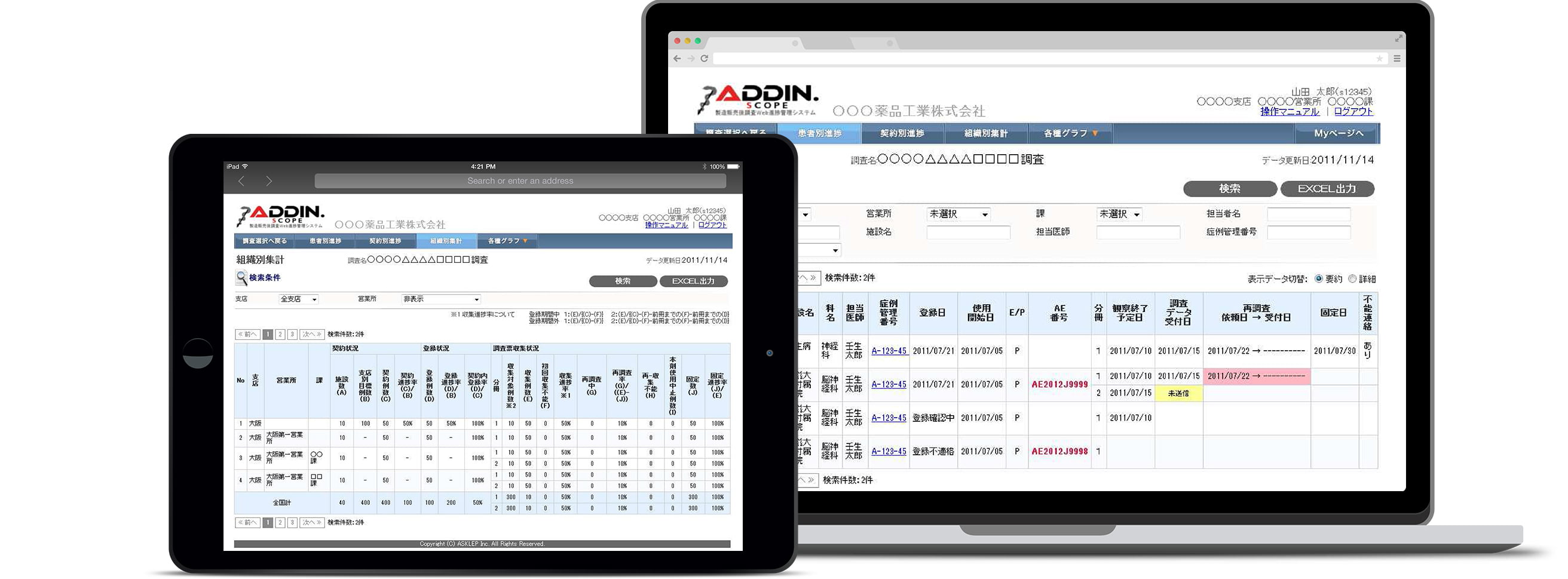
ADDIN.SCOPE- Progress Follow-up System -
Smooth implementation of Post-marketing Surveillance with our PMS platform
ADDIN.SCOPE is our web-based progress follow-up systems available via the internet. Using our system as your PMS platform provides smooth and reliable implementation of surveys from its ability for diverse data interactions. We offer a system best suited to satisfy the needs of our customers.
With ADDIN.SCOPE, the progress transition and passing ratio are checked by the department and visualized from the beginning of a survey.

|image scroll
Service Features
- Point 1.
- Cloud-type, web-based progress follow-up system with very little investment
- Point 2.
- Diverse data interactions and less stressful progress follow-up from the most effective use of EDC / CDMS data
- Point 3.
- User-friendly screens designed with simplicity. Quick and easy information sharing function is also available.
Diverse Data Interactions
- ADDIN series, CDMS, Other EDC system
- Data output from CSV files and progress data imports are conducted every day.
Optional Functions
- My Page
- Contracts information entry
- Fees management
- Tallying alert by department
Standard Functions
- Progress follow-up by patient, contract, and department
- Graph display (progress by month and department)
- Excel output
- Contract information interface
- EDC (ADDIN) survey information interface
- In-house and external EDC survey information interface
- Paper-based survey information interface
- Paper-based survey and CRF tracking (including receiving date)
- Multiple CRO capability (direct data uploads from CROs)
INTAGE TECHNOSPHERE, member of the Intage Group, offers POSTIER®/PMS a cloud based Progress Follow-up System for Post-Marketing Surveillance.
For further information onINTAGE Healthcare Inc.
If you have any questions or comments, please do not hesitate to contact us.
Contact UsINTAGE Healthcare’s Scope of Service
-
Marketing Research
From OTC products to ethical drugs and medical devices, we provide complete coverage of the healthcare market
- OTC Market Research
- Ethical Pharmaceutical Market Research
- Medical Device Research
- Quick Survey
- Global Research Service
-
Data Science Services
We contribute to the restructuring of a sustainable medical insurance system and the realization of optimal healthcare through various services based on data valuation concept.
- Health Economics and Outcomes Research (HEOR)
- DB Research
- Signal Management Service (Adverse Events Information Data Service)
- Drug Discovery Support
- Human Factors Engineering/Usability Engineering (HFE/UE)
-
CRO Services
We support customers to solve their issues with abundant achievements, knowledge, and advanced nature in the CRO business.
- Clinical Development Support Services
- PMS Support Services
- Monitoring
- Patient Registration
- EDC-enabled Data Management
- Biostatistics
- Medical Writing
- Auditing/Self-inspection
- Pharmacovigilance (GVP)
- DB Survey
- EDC Services
- ADDIN Series
- ePRO (Patient Reported Outcomes System)

